STATUS
In Commercial DevelopmentSTART DATE
2010The APAS Analysis Modules (AMs) are the intelligent image analysis software modules that facilitate use of the APAS® Independence instrument.
LBT’s first AM, the Urine AM, was successfully proven in a 10,000-patient clinical trial to screen for bacterial growth. Future AMs continue to be developed to enable screening of the most common pathogens encountered in clinical microbiology. Each AM released improves the clinical utility of the APAS Independence and increases its value for laboratories.
Challenges for Microbiology labs
70% of clinical decisions are based on in vitro diagnostic lab results.
Up to 85% of plates read are negative or show no significant growth, resulting in an inefficient use of resources.
Variability in plate reading with error rates 5.5-6.6%, and 12% for morphology.
There is a 9% vacancy rate in the US, and microbiologists’ average age is high (51 AUS / 42 US).
LBT Solution
The APAS® Independence was developed to assist microbiologists through the automation of culture plate screening and interpretation. The APAS Analysis Modules (AMs) are the intelligent image analysis software modules that work together with the APAS Independence to enable screening of the most common pathogens in a given clinical context.
A separate AM is developed for each of the most common specimen types received such as urine and infection control screening. This increases the volume of samples the APAS Independence can interpret, in turn increasing the clinical utility of the instrument for microbiologists by:
- detecting the presence of bacterial growth on an agar plate
- determining how much growth is present on the agar plate
- differentiating between common colony morphologies
- generating meaningful test results and reports for the sample
- alerting microbiologists where additional tests may be required
LBT currently has a number of AM modules in development to cover the most common clinical and non-clinical microbiology applications:
- Infection Control such as MRSA (Methicillin-resistant Staphylococcus aureus), VRE (vancomycin-resistant enterococci), CRE (Carbapenem-resistant Enterobacteriaceae)
- Sterile sites
- Sputum
- Antibiotic Sensitivity Testing (AST) and Direct Sensitivity Testing (DST)
- Food and Water samples – Total Plate Counts
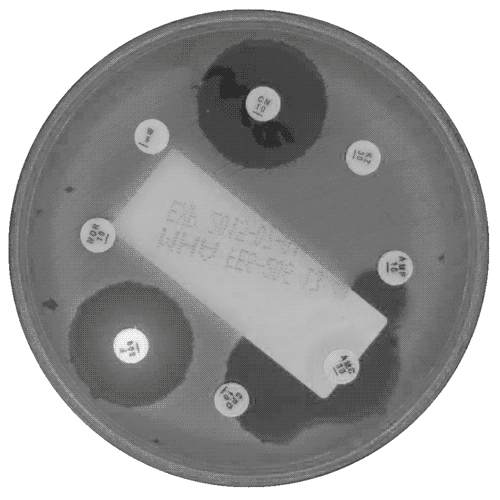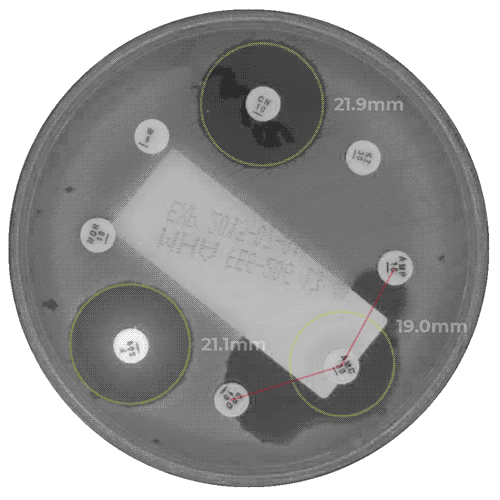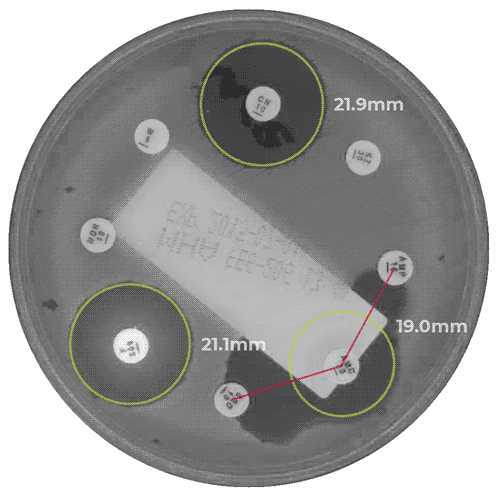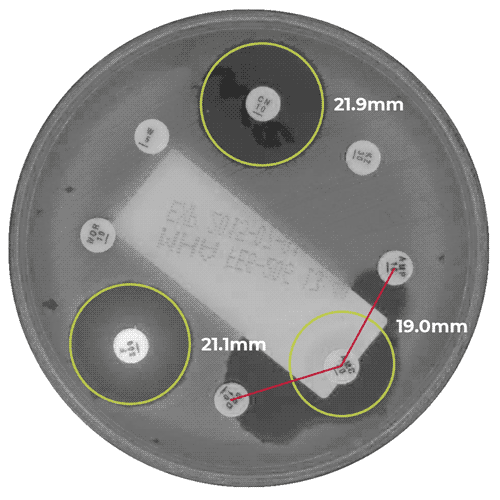
APAS AST Analysis Module for the measurement of zones of inhibition in antibiotic susceptibility testing