Reliable environmental monitoring plate reading powered by A.I.
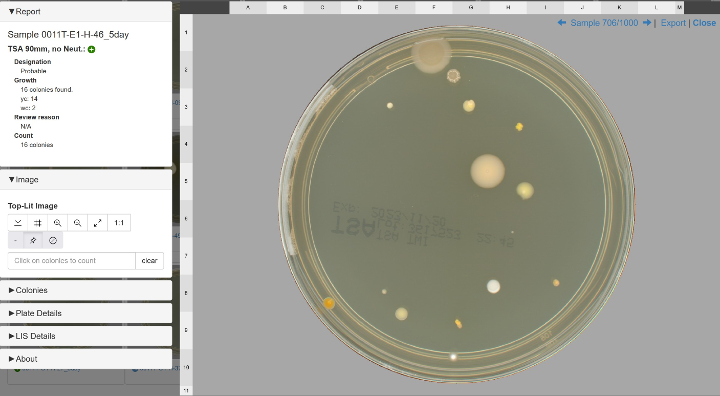
APAS PharmaQC provides automated imaging, analysis and interpretation of microbiology culture plates used in environmental monitoring. The cutting-edge technology utilises advanced imaging and sophisticated artificial intelligence algorithms to identify and count microbial growth on culture media delivering a greater level of traceability and data integrity to your environmental monitoring workflow.
Status: Available
Challenges in Environmental Monitoring
Microbial quality control is an essential activity for monitoring critical production environments where sterility is required to ensure the safety of drugs. Contamination control processes generate a large volume of environmental monitoring data that must be reviewed and interpreted by skilled microbiologists.
- Data Integrity – Manual counting and recording processes subject to human error
- Operational Inefficiency – Critical time spent performing repetitive labour-intensive tasks
- Variable Results – Interpretation inconsistencies between microbiologists
- Time Consuming – High volume of samples with no microbial growth (up to 90%)1
- High Regulatory Requirements – Contamination control strategy and Annex 1 requirements
- Validation Burden – Hurdles to implement new technologies and processes within controlled environments
1A Systematic Approach for the Evaluation, Validation, and Implementation of Automated Colony Counting Systems, Sven Deutschmann, Bill Carpenter, Caroline Duignan, et al., PDA Journal of Pharmaceutical Science and Technology 2022
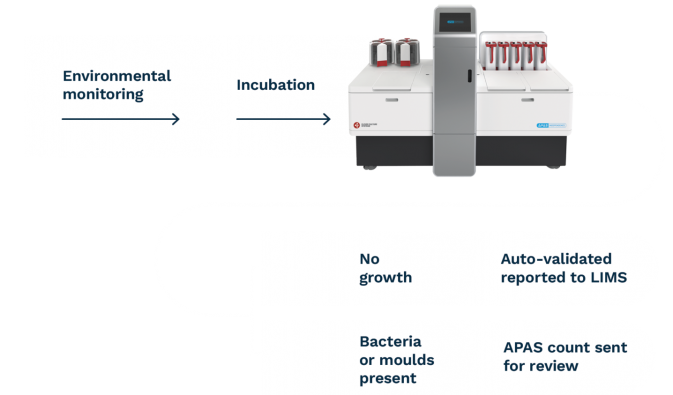
APAS PharmaQC Workflow
APAS Pharma saves time and enhances Microbial QC practices through automated imaging and interpretation of settle plates used in environmental monitoring. APAS Pharma integrates with existing work practices and is able to be validated across a range of standard 90mm Tryptone Soy Agar culture media.